
Cesium-137 (Cs-137) is a radioactive isotope of cesium with a half-life of about 30.17 years. It undergoes beta decay to produce barium-137m, which subsequently emits gamma radiation. Cs-137 is a byproduct of nuclear fission in reactors and is not naturally occurring. Due to its relatively long half-life and gamma emission, it is one of the most significant isotopes in terms of environmental and radiological impact following nuclear accidents.

Cs-137 has several practical applications. It is widely used in industrial radiography for material inspection and in the calibration of radiation detection equipment due to its well-defined gamma emissions. In medicine, Cs-137 is utilized in certain types of cancer treatment, specifically in brachytherapy for cervical cancer. It is also employed in research and as a radiation source in hydrology to measure soil erosion and sediment transport.

Cs-137 is primarily encountered in controlled environments such as medical facilities, research laboratories, and industrial settings. However, it is also found in the environment as a result of nuclear testing and accidents, such as the Chernobyl and Fukushima disasters. Contaminated areas may contain traces of Cs-137 in soil and water, where its persistence necessitates careful monitoring and remediation efforts.
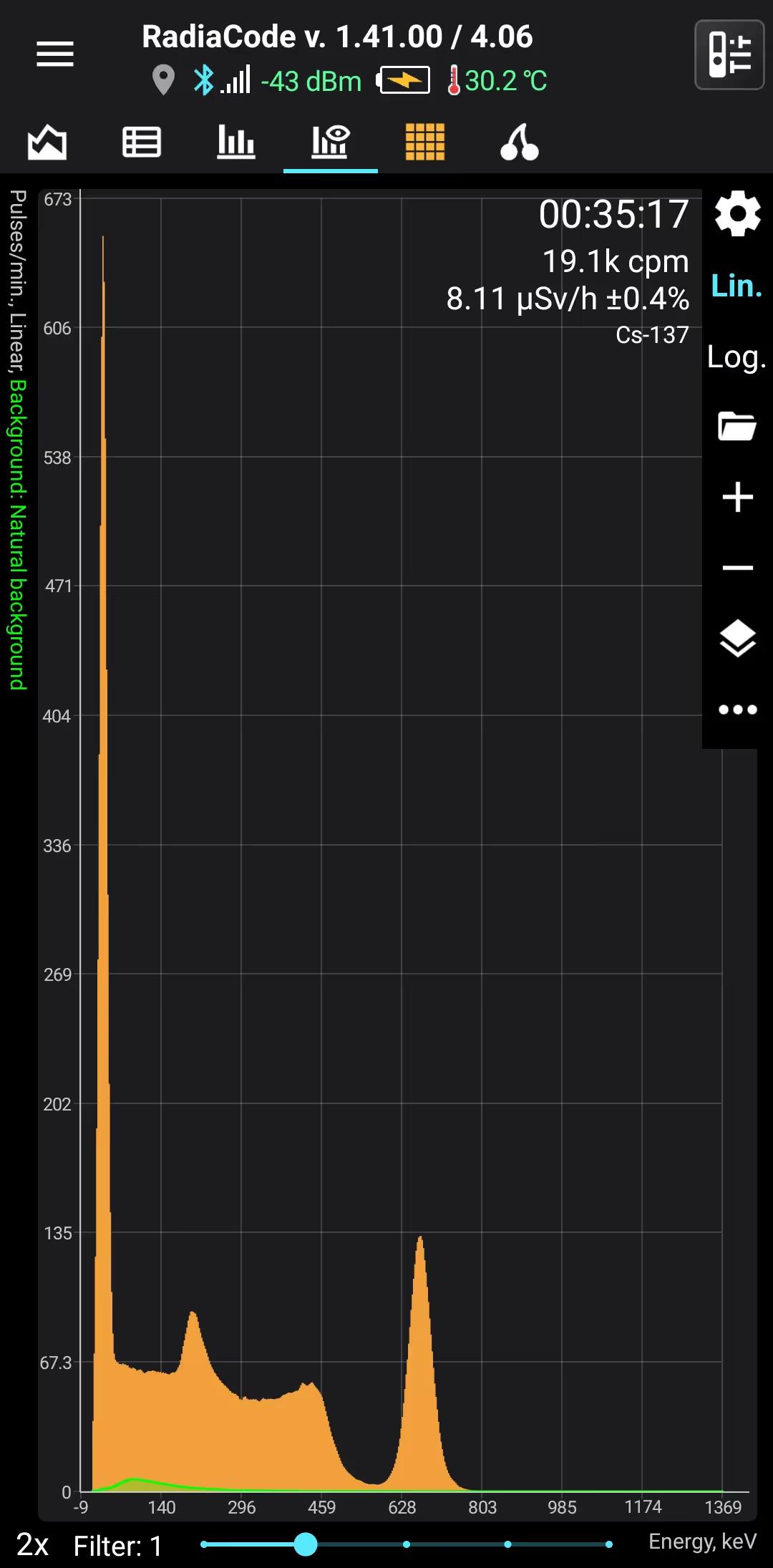
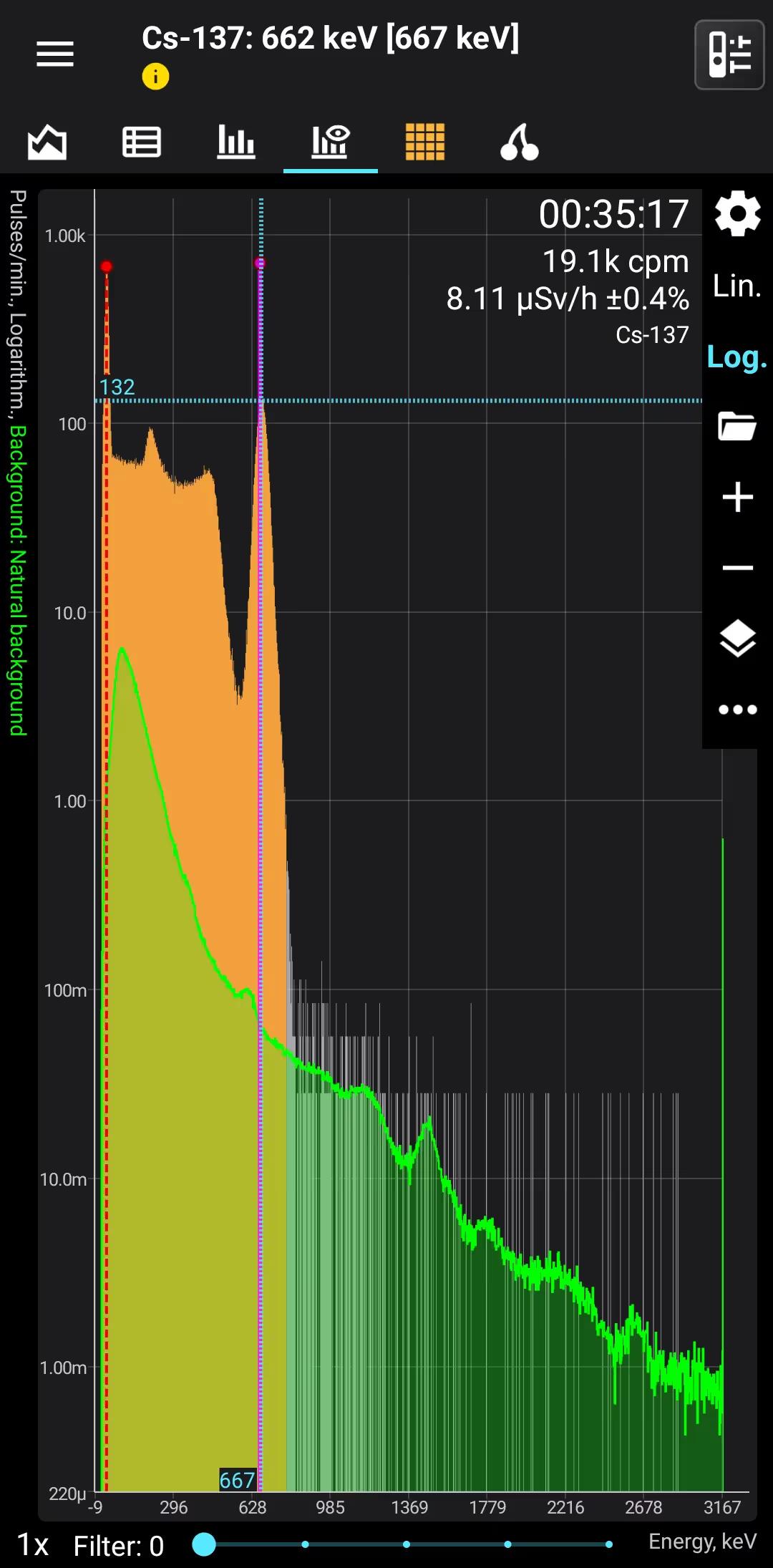
Cs-137
Cesium-137
Half-life: 30,16 years Main emission lines: 32, 662 keV
Decay mode Beta-
Beta-
| Avg. En., keV | Intensity, % | Decay En., keV |
| 174.32 | 94.7 | 514.03 |
| 416.26 | 5.3 | 1.176 |
Gamma
| Energy, keV | Intensity, % |
| 661.657 | 85.1 |
X-rays
| Energy, keV | Intensity, % |
| 32.193 | 3.67 |
| 31.816 | 1.99 |
| 36.304 - 37.350 | 1.35 |
| 36.304 - 36.660 | 1.08 |
| 3.956 - 5.975 | 0.91 |
| 37.249 - 37.261 | 0.27 |