
Iodine-123 (I-123) is a radioactive isotope of iodine with a half-life of approximately 13.2 hours. It decays by electron capture to tellurium-123, emitting gamma radiation with a characteristic energy of 159 keV, which is ideal for medical imaging. Iodine-123 is produced in a cyclotron by bombarding Xenon-124 with a proton. Xenon-124 either will absorb the proton and loses a proton and a neutron to form Xenon-123, or Xenon-124 will absorb the proton and loses two neutrons to form Caesium-123. Caesium will later decay into Xenon-123 while Xenon-123 will decay in Iodine-123.

I-123 is primarily used in nuclear medicine for diagnostic imaging. Its gamma emission properties make it highly suitable for single-photon emission computed tomography (SPECT) scans. It is commonly used in radiopharmaceuticals for imaging the thyroid gland, allowing physicians to assess thyroid function and detect disorders such as hyperthyroidism, hypothyroidism, and thyroid nodules. Additionally, I-123 is used in brain imaging to study neurotransmitter systems and to diagnose certain neurological disorders, such as Parkinson’s disease, through radiolabeled tracers like I-123 ioflupane (DaTSCAN).

I-123 does not occur naturally and must be produced in specialized facilities, such as cyclotrons. Due to its short half-life, it is typically prepared and distributed to medical centers shortly before use. I-123 is encountered in nuclear medicine departments, where it is administered to patients for diagnostic procedures. Its production, transportation, and application are tightly regulated to ensure safety and efficacy in clinical settings.
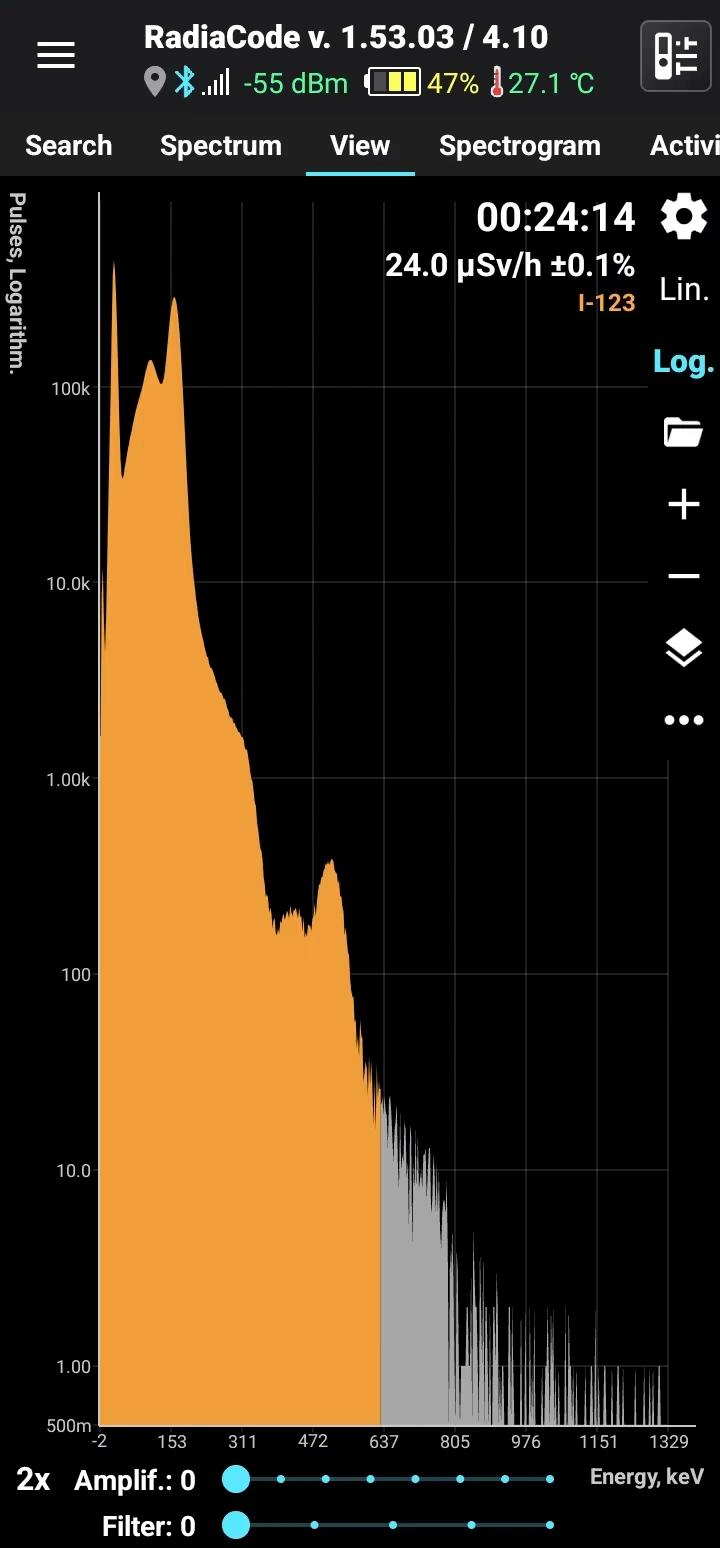
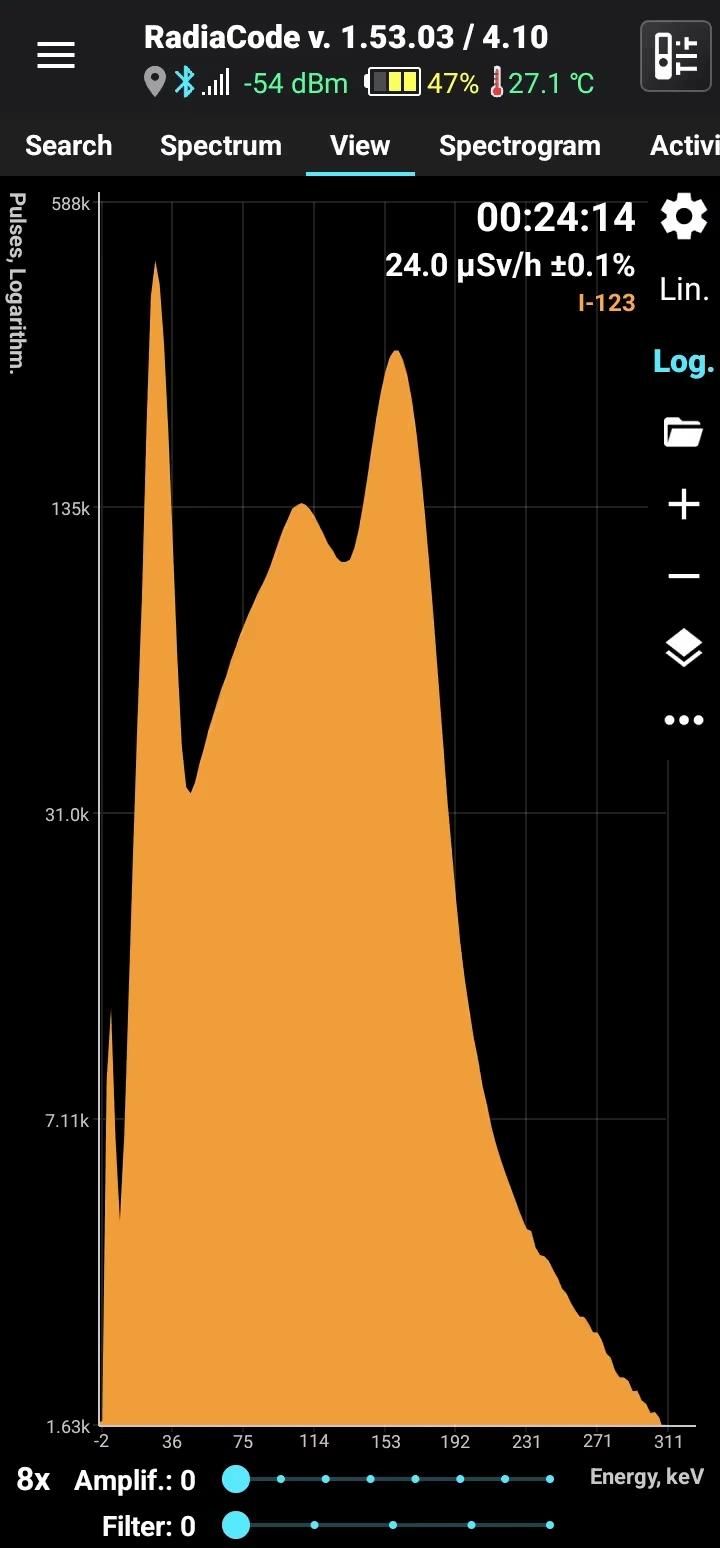
I-123
Iodine-123
Half-life: 13 hours Main emission lines: 27, 158 keV
Decay mode ec Beta+
Gamma
| Energy, keV | Intensity, % |
| 159.00 | 83.60 |
| 528.97 | 1.27 |
X-rays
| Energy, keV | Intensity, % |
| 27.473 | 45.9 |
| 27.202 | 24.63 |
| 30.944 - 31.774 | 15.97 |
| 30.944 - 31.242 | 13.12 |
| 3.335 - 4.936 | 9.0 |
| 31.693 - 31.703 | 2.85 |