
This isotope is almost always present in a mixture with other isotopes characteristic of the decay chain of uranium, radium, or radon. It can be found separately in objects that have been exposed to incredibly high levels of radon for a long time, for example, on the internal parts of watches where paint based on Radium-226 was used.
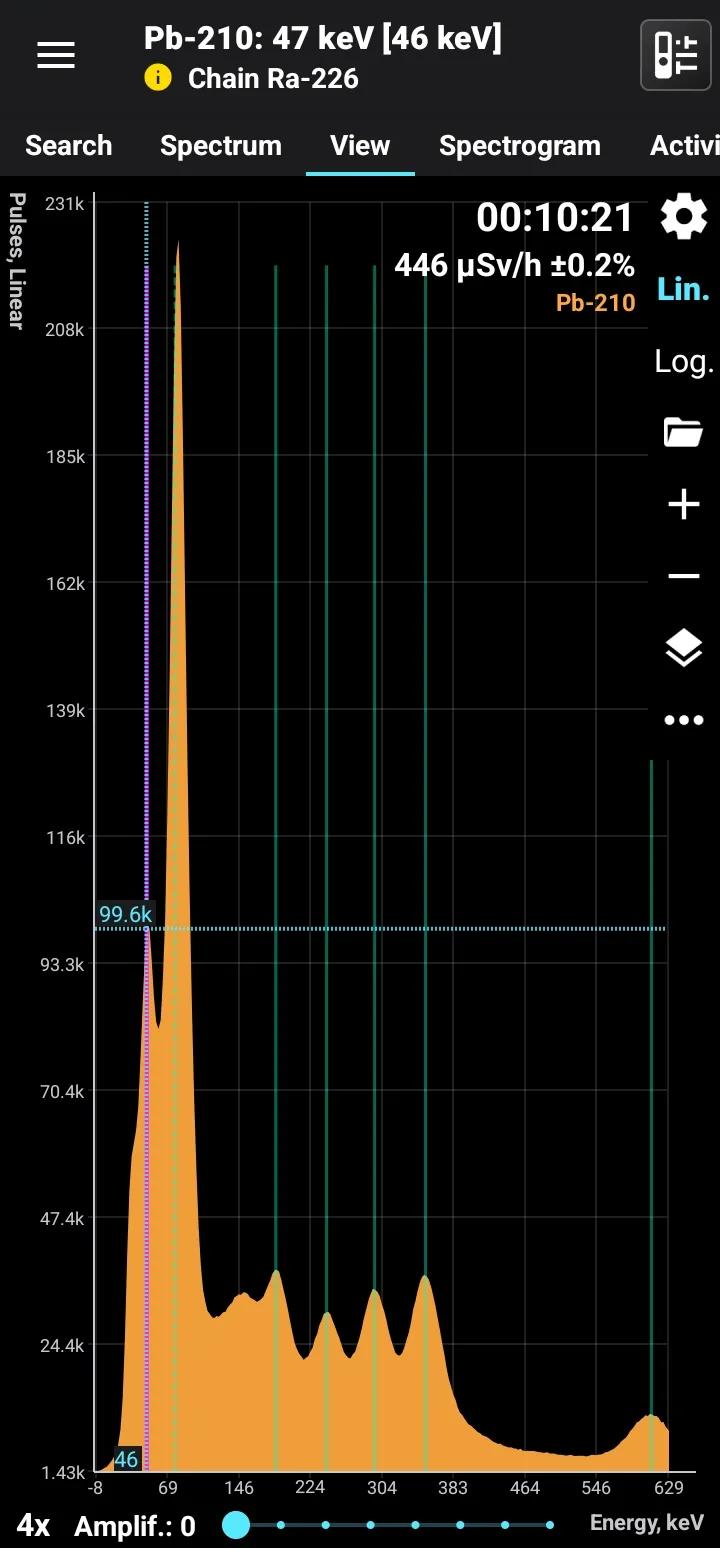
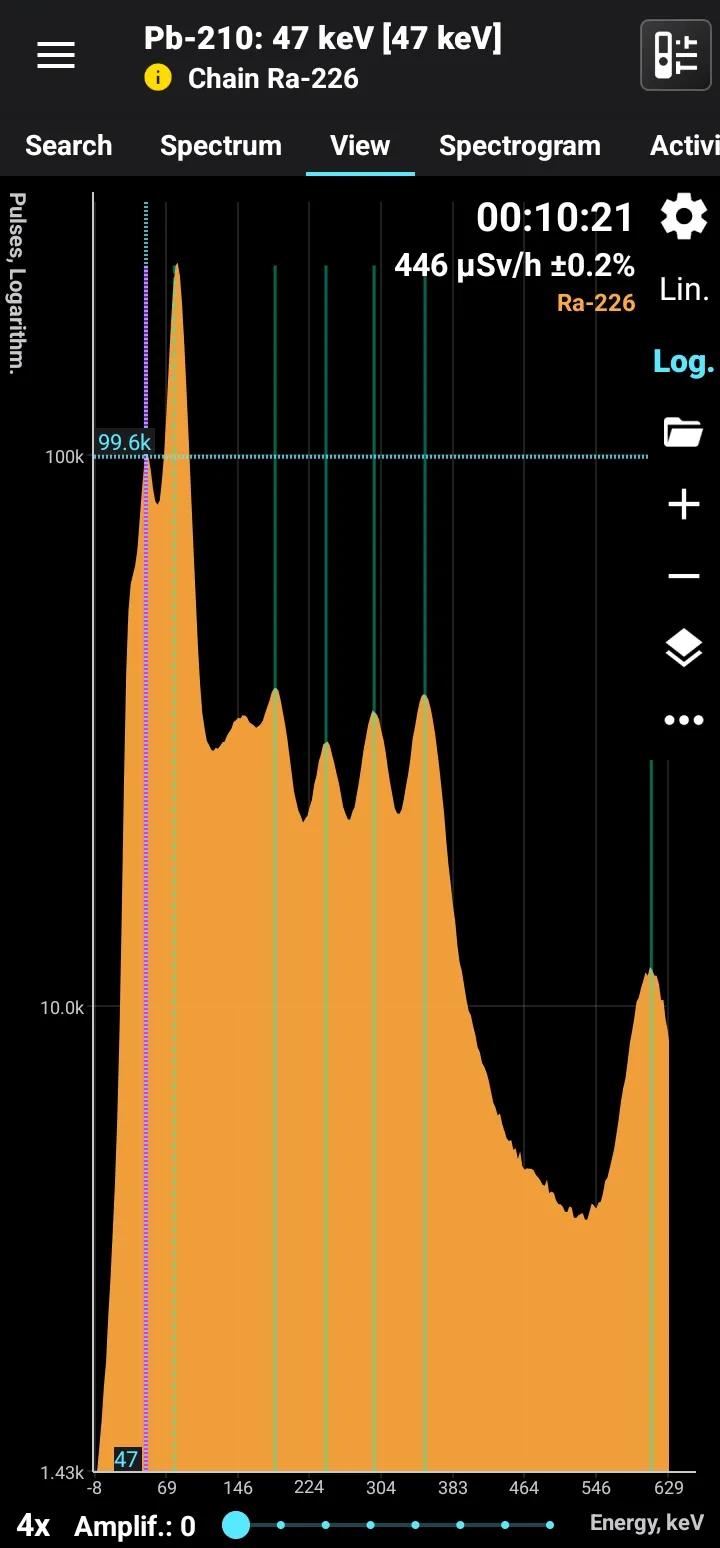
Pb-210 + Ra-226
Plumbum-210 + Radium-226
Natural α, β, γ radiation
Half-life: 22.3 years Main emission lines: 47 keV Decay chain: Ra-226 Related lines: 78, 186, 242, 295, 351, 609, 1120, 1760, 2200 keV
Decay mode Alpha
Alpha
| Energy, keV | Intensity, % |
| 3720 | 0.0000019 |
Decay mode Beta-
Beta-
| Energy, keV | Intensity, % | Decay En., % |
| 4.16 | 84 | (17.0) |
| 16.16 | 16 | (63.5) |
Gamma
| Energy, keV | Intensity, % |
| 46.539 | 4.25 |
X-rays
| Energy, keV | Intensity, % |
| 9.419 - 16.389 | 22.7 |