
Uranium without radium refers to uranium that has been chemically processed or purified to remove its decay products, including radium-226. This separation minimizes the presence of isotopes in the uranium decay chain, particularly those that contribute to gamma radiation. The remaining uranium primarily consists of its natural isotopes: uranium-238 (~99.3%), uranium-235 (~0.7%), and trace uranium-234 (~0.005%). These isotopes primarily emit alpha particles, and the absence of radium significantly reduces the associated gamma radiation from uranium’s decay chain.

Uranium (without radium impurities) is found in any uranium glass, Red Fiesta glaze, or reagents with uranium compounds.
Over time, radium will begin to accumulate, but it would take millions of years for the spectrum to take on the appearance of natural uranium.
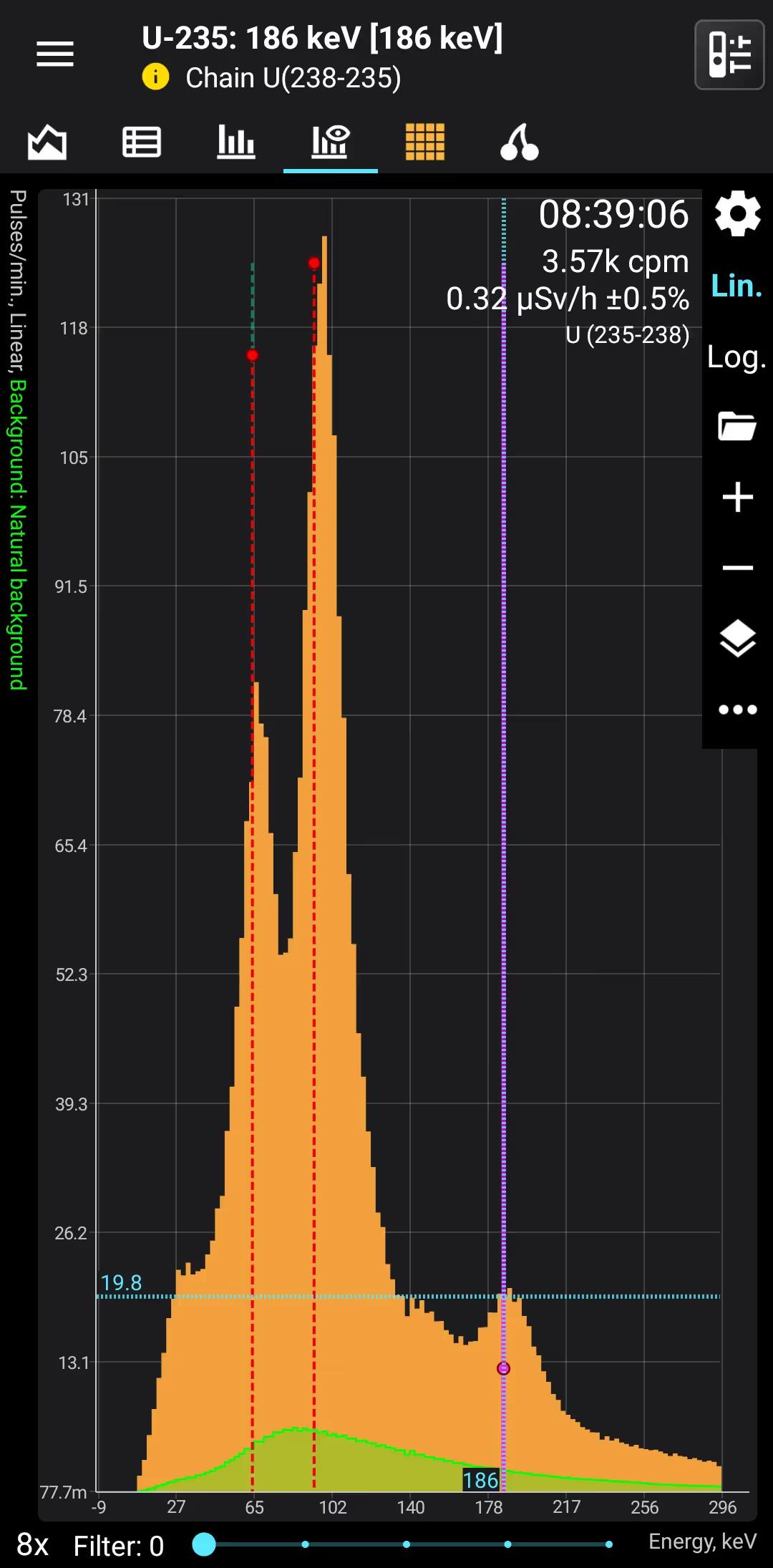
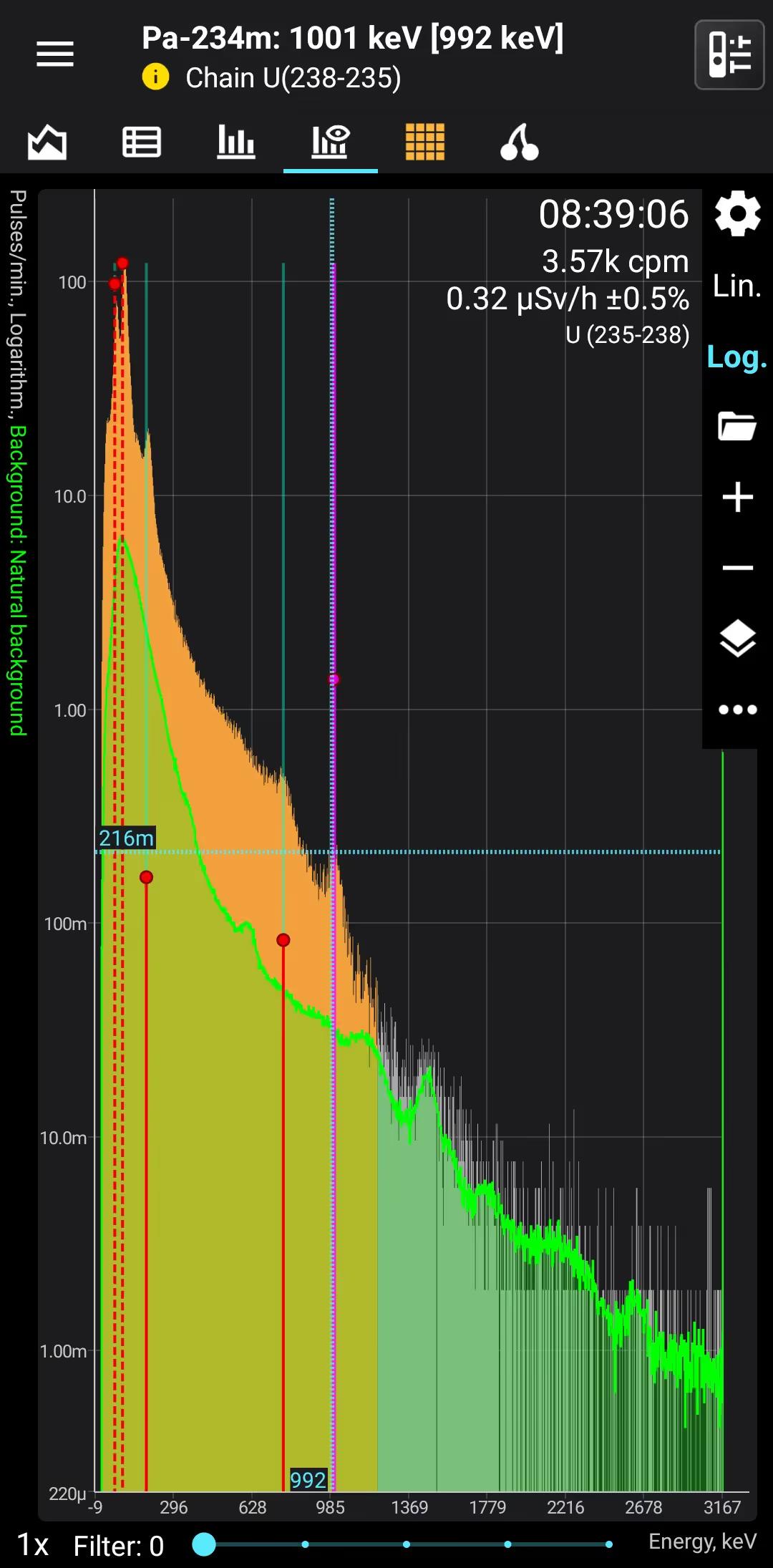
U (235-238)
Uranium 235-238
Half-life: 4,5 billion years Main emission lines: 65, 95, 185, 750, 1001 keV
U-235. Decay mode Alpha
Alpha
| Energy, keV | Intensity, % |
| 4395.7 | 57.8 |
| 4364.6 | 18.94 |
| 4216.0 | 6.10 |
| 4597.7 | 4.77 |
| 4556.5 | 3.82 |
| 4323.2 | 3.58 |
| 4415.1 | 3.09 |
| 4502.9 | 1.28 |
Gamma
| Energy, keV | Intensity, % |
| 185.713 | 57.2 |
| 143.765 | 10.93 |
| 163.357 | 5.07 |
| 205.311 | 5.03 |
| 109.18 | 1.64 |
| 202.110 | 1.07 |
| 194.942 | 0.637 |
| 19.55 | 0.583 |
X-rays
| Energy, keV | Intensity, % |
| 11.118 - 20.450 | 27.7 |
| 93.347 | 4.8 |
| 89.954 | 3.0 |
| 104.817 - 108.971 | 2.30 |
| 104.817 - 106.315 | 1.72 |
| 108.479 - 108.680 | 0.58 |
U-238. Decay mode Alpha
Alpha
| Energy, keV | Intensity, % |
| 4198 | 79 |
| 4151 | 21 |
Gamma
| Energy, keV | Intensity, % |
| 49.55 | 0.064 |
| 113.5 | 0.0102 |
X-rays
| Energy, keV | Intensity, % |
| 11.118 - 20.450 | 7.3 |